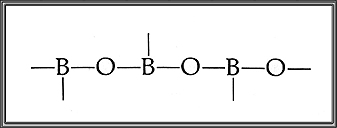
Boric acid, H3BO3. This white solid, also called boracic, or orthoboric, acid, is obtained by treating a concentrated solution of borax with sulfuric or hydrochloric acid. Boric acid is commonly used as a mild antiseptic for burns and surface wounds and is a major ingredient in eye lotions. Among its other important applications are its use as a fire-retardant in fabrics, in solutions for electroplating nickel or for tanning leather, and as a major constituent in catalysts for numerous organic chemical reactions. Like Silicon, Boron forms large molecules in which oxygen atoms occupy alternate positions.
The repeating structure of Boric Acid
Insects die by ingesting boric acid and borate salts. Borate salts are abrasive to the insect's exo-skeleton. It is mostly used as a bait mixed with minced liver to treat Pharoahs Ants, although these days juvenile hormone growth regulators tend to be the norm.
LD50/LC50:
Boric acid is very low in toxicity when ingested. The acute oral LD50 in mice is 3450 mg/kg and for rats ranges from 2669 - 5140 mg/kg. The LC50 values for mice for inhaled boron compounds range from 0.89 - 21.1 mg/L indicating very low to low inhalation toxicity. The proposed potential lethal boric acid doses are 3-6 g for infants and 15-20 g for adults.