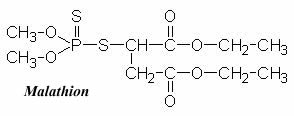
Malathion Malathion is a nonsystemic, wide-spectrum organophosphate insecticide. It was one of the earliest organophosphate insecticides developed (introduced in 1950). Malathion is suited for the control of sucking and chewing insects on fruits and vegetables, and is also used to control mosquitoes, flies, household insects, animal parasites (ectoparasites), and head and body lice. Malathion may also be found in formulations with many other pesticides.IUPAC Name: diethyl (dimethoxythiophosphorylthio)succinate
or
S-1,2-bis(ethoxycarbonyl)ethyl O,O-dimethyl phosphorodithioateChemical Formula: C10H19O6PS2
LD50/LC50: Malathion is slightly toxic via the oral route, with reported oral LD50 values of 1000 mg/kg to greater than 10,000 mg/kg in the rat, and 400 mg/kg to greater than 4000 mg/kg in the mouse. It is also slightly toxic via the dermal route, with reported dermal LD50 values of greater than 4000 mg/kg in rats.
It has been reported that single doses of malathion may affect immune system response. Symptoms of acute exposure to organophosphate or cholinesterase-inhibiting compounds may include the following: numbness, tingling sensations, incoordination, headache, dizziness, tremor, nausea, abdominal cramps, sweating, blurred vision, difficulty breathing or respiratory depression, and slow heartbeat. Very high doses may result in unconsciousness, incontinence, and convulsions or fatality. The acute effects of malathion depend on product purity and the route of exposure.
Other factors which may influence the observed toxicity of malathion include the amount of protein in the diet and gender. As protein intake decreased, malathion was increasingly toxic to the rats. Malathion has been shown to have different toxicities in male and female rats and humans due to metabolism, storage, and excretion differences between the sexes, with females being much more susceptible than males. Numerous malathion poisoning incidents have occurred among pesticide workers and small children through accidental exposure. In one reported case of malathion poisoning, an infant exhibited severe signs of cholinesterase inhibition after exposure to an aerosol bomb containing 0.5% malathion.
Effects on birds: Malathion is moderately toxic to birds. The reported acute oral LD50 values are: in mallards, 1485 mg/kg; in pheasants, 167 mg/kg; in blackbirds and starlings, over 100 mg/kg; and in chickens, 525 mg/kg.
Effects on aquatic organisms: Malathion has a wide range of toxicities in fish, extending from very highly toxic in the walleye (96-hour LC50 of 0.06 mg/L) to highly toxic in brown trout (0.1 mg/L) and slightly toxic in goldfish (10.7 mg/L). Malathion is highly toxic to aquatic invertebrates and to the aquatic stages of amphibians. Because of its very short half-life, malathion is not expected to bioconcentrate in aquatic organisms. However, brown shrimp showed an average concentration of 869 and 959 times the ambient water concentration in two separate samples.
Effects on other organisms: The compound is highly toxic to honeybees.
Breakdown in soil and groundwater: Malathion is of low persistence in soil with reported field half-lives of 1 to 25 days. Degradation in soil is rapid and related to the degree of soil binding. Breakdown occurs by a combination of biological degradation and nonbiological reaction with water. If released to the atmosphere, malathion will break down rapidly in sunlight, with a reported half-life in air of about 1.5 days. It is moderately bound to soils, and is soluble in water, so it may pose a risk of groundwater or surface water contamination in situations which may be less conducive to breakdown.
Breakdown in water: In raw river water, the half-life is less than 1 week, whereas malathion remained stable in distilled water for 3 weeks. Applied at 1 to 6 lb/acre in log ponds for mosquito control, it was effective for 2.5 to 6 weeks. In sterile seawater, the degradation increases with increased salinity. The breakdown products in water are mono- and dicarboxylic acids.
Breakdown in vegetation: Residues were found mainly associated with areas of high lipid content in the plant. Increased moisture content increased degradation.
Physical Properties:
- Appearance: Technical malathion is a clear, amber liquid at room temperature.
- Chemical Name: diethyl (dimethoxy thiophosphorylthio) succinate.
- CAS Number: 121-75-5.
- Molecular Weight: 330.36.
- Water Solubility: 130 mg/L.
- Solubility in Other Solvents: v.s. in most organic solvents.
- Melting Point: 2.85 C.
- Vapor Pressure: 5.3 mPa @ 30 C.
- Partition Coefficient: 2.7482.
- Adsorption Coefficient: 1800.
Sources: Oregon University and World Health Organisation