DDT got it's name from an old and imprecise name DichloroDiphenylTrichloroethane and was the insecticide to end all insecticides when it came out. DDT is an organochlorine insecticide which was discovered and first synthesised in 1874 by a chemist called Zeidler. Later another scientist, Mueller, discovered DDT's insecticidal properties in 1939. DDT is effective against many organisms, but its most spectacular success has been in the control of the Anopheles mosquito, which transmits malaria. Malaria has been the scourge of mankind for centuries. According to the World health Organisation, malaria is still the chief cause of human death in the world, aside from natural causes. The disease acquired its name in ancient Rome ( L. mala, bad; aria, air), where it was believed to be the result of bad air in the city. It is actually caused by a parasite of the Plasmodium family which infects and ruptures erythrocytes in the blood stream. The organism has a complex life cycle requiring both vertebrate and invertebrate. Humans are infected with the sporozoites of the organism which are injected into the bloodstream by the bite of an infected mosquito. Although malaria may be treated, the most effective way of controlling it is to eliminate the insect vector which is essential for its transmission. DDT is especially effective for this purpose, and malaria had essentially been eliminated from large areas of the world through its use...signs are now that it is on the increase again. It has been estimated that because of the efficacy of DDT in checking malaria and other mosquito borne diseases (yellow fever, encephalitis), more than 75 million deaths have been averted. A striking example is Sri Lanka ( used to be Ceylon). In 1934 - 35, there were 1.5 million cases of malaria resulting in 80,000 deaths. After an intensive mosquito-abatement program using DDT, malaria effectively dissappeared and there were only 17 cases reported in 1963. When the use of DDT was discontinued in Sri Lanka, malaria rebounded , and there were over 600,000 cases reported in 1968 and the first quarter of 1969In spite of its obvious value in combatting diseases like malaria, DDT has been abused. It is a "hard" inseciticide, in that its residues accumulate in the environment. Although it is not especially toxic to mammals ( The fatal human dose is 500 mg/kg of body weight, about 35 grams for a 150lb person), it is concentrated by lower organisms such as plankton and accumulates in the fatty tissues of fish and birds. The toxicity of DDT was first noted in 1949 by the Fish and Wildlife Service in America, and following abuse by crop sprayers, was eventually withdrawn in 1972 by the then Environmental Protection Agency.
As animals on the lower end of the food chain are eaten by those higher up, DDT becomes more and more concentrated the higher you go. This continues until the primary predator is reached, who will then receive the highest dose. DDT is highly persistent in the soil and can last from 2 - 15 years, not too bad some people might say, but when you look at the half-life in an aquatic environment, this can be about 150 years, one half-life being that time to degrade by 50%. DDT is highly acutely toxic to fish affecting membrane funtion and enzyme systems. Atlantic salmon fry were found to be affected at concentrations of 50 - 100 µg/L, suffering from balance problems and impaired behavioural development. At the same time aquatic invertabrates and amphibians are also affected allbeit to a very slighter extent.
It not only acts as a stomach poison but also as a contact insecticide, and was remarkable for its high toxicity to insects at low rates of application. DDT affects the nervouse system by interfering with normal nerve impulses. DDT causes the nerve cells to repeatedly generate an impulse, this accounts for the tremors seen in exposed animals.
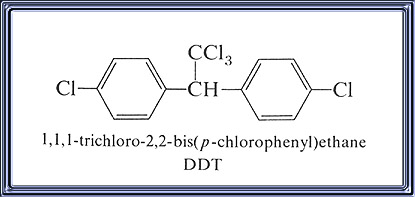
IUPAC Name: 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane
Chemical Formula: C14H9Cl5
LD50/LC50: DDT is moderately to slightly toxic to studied mammalian species via the oral route. Reported oral LD50s range from 113 to 800 mg/kg in rats (79,73); 150-300 mg/kg in mice (79); 300 mg/kg in guinea pigs; 400 mg/kg in rabbits; 500-750 mg/kg in dogs and greater than 1,000 mg/kg in sheep and goats.
Effects on Birds: DDT may be slightly toxic to practically non-toxic to birds. Reported dietary LD50s range from greater than 2,240 mg/kg in mallard, 841 mg/kg in Japanese quail and 1,334 mg/kg in pheasant. In birds, exposure to DDT occurs mainly through the food web through predation on aquatic and/or terrestrial species having body burdens of DDT, such as fish, earthworms and other birds. There has been much concern over chronic exposure of bird species to DDT and effects on reproduction, especially eggshell thinning and embryo deaths.
Effects on Aquatic Species: DDT is very highly toxic to many aquatic invertebrate species. Reported 96-hour LC50s in various aquatic invertebrates (e.g., stoneflies, midges, crayfish, sow bugs) range from 0.18 ug/L to 7.0 ug/L, and 48-hour LC50s are 4.7 ug/L for daphnids and 15 ug/L for sea shrimp. Other reported 96-hour LC50s for various aquatic invertebrate species are from 1.8 ug/L to 54 ug/L. DDT is very highly toxic to fish species as well. Reported 96-hour LC50s are less than 10 ug/L in rainbow trout (8.7 ug/L), northern pike (2.7 ug/L). DDT may be moderately toxic to some amphibian species and larval stages are probably more susceptible than adults. In addition to acute toxic effects, DDT may bioaccumulate significantly in fish and other aquatic species, leading to long-term exposure. This occurs mainly through uptake from sediment and water into aquatic flora and fauna, and also fish. Fish uptake of DDT from the water will be size-dependent with smaller fish taking up relatively more than larger fish. A half-time for elimination of DDT from rainbow trout was estimated to be 160 days. The reported bioconcentration factor for DDT is 1,000 to 1,000,000 in various aquatic species, and bioaccumulation may occur in some species at very low environmental concentrations. Bioaccumulation may also result in exposure to species which prey on fish or other aquatic organisms (e.g., birds of prey).
Effects on Other Animals (Nontarget species): Earthworms are not susceptible to acute effects of DDT and its metabolites at levels higher than those likely to be found in the environment, but they may serve as an exposure source to species that feed on them. DDT is non-toxic to bees; the reported topical LD50 for DDT in honeybees is 27 ug/bee. Laboratory studies indicate that bats may be affected by DDT released from stored body fat during long migratory periods.
Breakdown in Soil and Groundwater: DDT is very highly persistent in the environment, with a reported half life of between 2-15 years and is immobile in most soils. Routes of loss and degradation include runoff, volatilization, photolysis and biodegradation (aerobic and anaerobic). These processes generally occur only very slowly. Breakdown products in the soil environment are DDE and DDD, which are also highly persistent and have similar chemical and physical properties. Due to its extremely low solubility in water, DDT will be retained to a greater degree by soils and soil fractions with higher proportions of soil organic matter. It may accumulate in the top soil layer in situations where heavy applications are (or were) made annually; e.g., for apples. Generally DDT is tightly sorbed by soil organic matter, but it (along with its metabolites) has been detected in many locations in soil and groundwater where it may be available to organisms. This is probably due to its high persistence; although it is immobile or only very slightly mobile, over very long periods of time it may be able to eventually leach into groundwater, especially in soils with little soil organic matter. Residues at the surface of the soil are much more likely to be broken down or otherwise dissipated than those below several inches.
Breakdown of Chemical in Surface Water: DDT may reach surface waters primarily by runoff, atmospheric transport, drift, or by direct application (e.g. to control mosquito-borne malaria). The reported half-life for DDT in the water environment is 56 days in lake water and approximately 28 days in river water. The main pathways for loss are volatilization, photodegradation, adsorption to water-borne particulates and sedimentation. Aquatic organisms, as noted above, also readily take up and store DDT and its metabolites. Field and laboratory studies in the United Kingdom demonstrated that very little breakdown of DDT occurred in estuary sediments over the course of 46 days. DDT has been widely detected in ambient surface water sampling in the United States at a median level of 1 ng/L (part per trillion).
Breakdown of Chemical in Vegetation: DDT does not appear to be taken up or stored by plants to a great extent. It was not translocated into alfalfa or soybean plants, and only trace amounts of DDT or its metabolites were observed in carrots, radishes and turnips all grown in DDT-treated soils. Some accumulation was reported in grain, maize and riceplants, but little translocation occured and residues were located primarily in the roots.
Physical Properties:
- Appearance: The physical appearance of technical product p,pÕ-DDT is a waxy solid, although in its pure form it consists of colorless crystals.
- Chemical Name: 1,1'-(2,2,2-trichloroethylidene)bis[4-chlorobenzene]; 1,1,1- trichloro-2,2-bis(4-chlorophenyl) ethane.
- CAS Number: 50-29-3.)
- Molecular Weight: 354.51.
- Water Solubility: < 1 mg/L @ 20°C
- Solubility in Other Solvents: cyclohexanone v.s., dioxane v.s., benzene v.s., xylene v.s., trichloroethylene v.s., dichloromethane v.s., acetone v.s., chloroform v.s., diethyl ether v.s., ethanol s. and methanol s..
- Melting Point: 108.5-109°C.
- Vapor Pressure: 0.025 mPa @ 25°C.
- Partition Coefficient: Not available.
- Adsorption Coefficient: 100,000.
References:
- World Health Organisation.
- USA Environmental Protection Agency.
- Belstein.
- Oregon University.