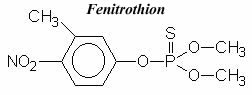
Fenitrothion Fenitrothion is a contact insecticide and selective acaricide of low ovicidal properties. It belongs to the organophosphate family of insecticides. It is considered a cholinesterase inhibitor. It may also be used as a fly, mosquito, and cockroach residual contact spray for farms and public health programs. Fenitrothion is also effective against household insects and all of the nuisance insects listed by the World Health Organization. Its effectiveness as a vector control agent for malaria is confirmed by the World Health Organization. Fenitrothion is non-systemic, and non-persistent. Fenitrothion was introduced in 1959 by both Sumitomo Chemical Company and Bayer Leverkusen and later by American Cyanamid Company. Fenitrothion is far less toxic than parathion with a range of insecticidal activity that is very similar and is similar enough in structure to be produced in the same factories. The difference in precursor chemicals might make it somewhat more expensive, but it is heavily used in other countries, including Japan, where parathion has been banned. Fenitrothion comes in dust, emulsifiable concentrate, flowable, fogging concentrate, granules, ULV, oil-based liquid spray, and wettable powder formulations. It is available as a 95% concentrate, 50% emulsifiable concentrate, 40% and 50% wettable powder and 2%, 2.5%, 3% and 5% dusts. It is compatible with other neutral insecticides.IUPAC name: O,O-dimethyl O-4-nitro-m-tolyl phosphorothioate
Chemical Formula: C9H12NO5PS
LD50/LC50: The acute toxicity of fenitrothion to mammals is considered to be low. The acute oral LD50 for rats ranges between 250-800 mg/kg; 715-870 mg/kg for mice; and 500 mg/kg for guinea pigs. The acute dermal LD50 for rats is >890 mg/kg and >3,000 mg/kg for mice. The acute inhalation LC50 in rats was reported to be 5.0 mg/l. The oral acute toxicity for cats was 142 mg/kg. Chronic symptoms in humans include: general malaise, fatigue, headache, loss of memory and ability to concentrate, anorexia, nausea, thirst, loss of weight, cramps, muscular weakness and tremors.
Effects on Birds: Negative results were observed in studies on delayed neurotoxicity in hens. The oral LD50 for chickens is reported as 28 mg/kg. Fenitrothion was found to be highly toxic to upland gamebirds and slightly toxic to waterfowl (acute oral toxicity value to quail and mallards was determined to be 23.6 mg/kg and 1,190 mg/kg, respectively). The LC50 for pheasants was 450 to 500 ppm in diets of 2-week-old birds when fed fenitrothion-treated feed for 5 days, followed by untreated feed for 3 days.
Effects on Aquatic Organisms: The time for achieving the highest levels of uptake and the extent of retention of organophosphate residues by fish was directly related to the extent of persistence of a compound in water. Fenitrothion (4.9 mg/kg) persisted longer than 4 weeks in fish. Fenitrothion is considered somewhat toxic to fish. The 96-hour LC50 was 1.7 ppm for brook trout; moderately toxic to both warmwater and coldwater fish. The chronic toxicity of fenitrothion to fish is considered low. The 48-hour LC50 values for carp ranged between 2.0 mg/l and 4.1 mg/l. In a study on the acute toxicity of fenitrothion to rainbow trout, embryos were found to be the least sensitive, the sacfry stage was intermediate, and fingerlings and adults were the most sensitive. The toxicity of fenitrothion to rainbow trout increased with increasing temperature. The sublethal effects of fenitrothion exposure on fish include:
Morpho Anatomical Changes: Swelling of the abdomen of fathead minnows occured. Young Atlantic salmon exposed to 1 mg/l swam with distended fins.
Behavioral Changes: There was a pronounced decline in various agonistic behaviors (chasing, vacating, nipping, etc.) within 2 hours of exposure to several concentrations of fenitrothion.
Biochemical Changes: Acetylcholinesterase activity was inhibited 13% to 25% after various sublethal concentrations of fenitrothion. Cholinesterase activity in the erythrocytes, gills, heart, and serum of rainbow trout was reduced within 1 hour after exposure to fenitrothion.
Effect on Growth: Orally administrated fenitrothion had no effect on the growth of rainbow trout. The compound is considered very toxic to crustaceans and aquatic insects and has a medium toxicity to aquatic worms. A freshwater invertebrate toxicity (48-hour or 96-hour EC50) reported fenitrothion to be very highly toxic to aquatic invertebrates (3 ppb for Gammarus fasciatus ).
Effects on Other Animals (Nontarget species): There is sufficient information to characterize fenitrothion as highly toxic to honeybees (acute toxicity value = 0.383 micrograms/bee) when bees are exposed to direct treatment or to dried residues on foliage. Fenitrothion is considered toxic to spider mites with long residual action.
Breakdown of Chemical in Soil and Groundwater: Preliminary data indicates fenitrothion degrades fairly rapidly in soil with a half-life of less than one week in non-sterile muck, sandy loam soils. The compound is intermediately mobile in a variety of soils ranging from sandy loam to clay.
Breakdown of Chemical in Vegetation: Damage to cabbage and fruit is possible only if the application dose is exceeded. Fenitrothion has been know to be phytotoxic to cotton, Brassica crops, and certain fruit crops when high rates were applied. Certain apple varieties may be russeted. In a study conducted by FAO/WHO, about 50% of 32P-labelled fenitrothion sprayed on rice plants penetrated into the tissues in 24 hours. At the end of this period only 10% was left, indicating rapid decomposition.
Breakdown of Chemical in Air: An experiment was carried out in a vacant dormitory building in order to establish the airborne residue of concentrations of seven pesticides used for cockroach control. Airborne concentrations of fenitrothion on the day of application were 3 micrograms/cubic meter. All were below 0.7 micrograms/cubic meter by the third day after application. The airborne concentrations correlated well with the vapor pressures of the various pesticides
Physical Properties:
- Appearance: Pure material forms a yellowish brown liquid with an unpleasant odor (2, 125)
- Chemical Name: O,O-dimethyl O-4-nitro-m-tolyl phosphorothioate (IUPAC), O,O-dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate (CA), O,O-dimethyl O-(3-methyl-4-nitrophenyl) thiophosphate.
- CAS Number: 122-14-5.
- Molecular Weight: 277.25.
- Water Solubility: In water at 20 degrees C, 30 mg/l; at 30 degrees C, 14 mg/l water; nearly insoluble in water; insoluble in water.
- Solubility in Other Solvents: Readily soluble in common organic solvents, e.g. acetone, alcohol, benzene and chlorinated hydrocarbons. dichloromethane, 2-propanol, toluene. Hardly soluble in n-hexane. Soluble in ethers, methanol, xylene, ketones, esters, and aromatic hydrocarbons. Low solubility in alaphatic hydrocarbons. At 20 -25 degrees C, > 1 kg/kg dichloromethane, methanol and xylene, 42 g/kg haxane, 0.1 - 1.0 kg/kg propan-2-ol. It is hydrolyzed by alkali; at 30 degrees C, 50% loss occurs in 4.5 hours in 10M sodium hydroxide.
- Melting Point: 0.3 degrees C.
- Vapor Pressure: 7 x 10 to the minus 5 mbar at 20 degrees C (13);18 mPa at 20 degrees C.
- Partition Coefficient: 2380.
- Adsorption Coefficient: Not Available.
- Stability: Fenitrothion is completely stable for two years if stored at temperatures between 20 and 25 degrees C. Storage temperature should not exceed 40 degrees C. It is unstable in alkaline media. The thermal stability of this compound is low, and when it is heated above 100 degrees C it undergoes Pishchemuka isomerization and may decompose explosively. It must be stored in enameled, aluminum or glass containers. Iron promotes decomposition of fenitrothion.
- Specific gravity: 1.3227; 1.32-1.34; 1.3084 at 20 degrees C.
- Boiling point: 109 degrees C at 0.13 mbar; 164 degrees C at 1.3 mbar (13). 140-145 degrees C/0.1 mmHg. 244 degrees F (118 degrees C) at 0.05 mmHg (113). 118 degrees C at 0.01 mmHg.
- Flashpoint: 166 degrees C (closed cup).
- Volatility: 0.09 mg/m3.
****Warning****
When spraying anything which resembles Terrazzo tiling always do a test spray before committing yourself. Fenitrothion has a tendency to stain this type of tile yellow, this reaction is not reversible.
Sources include: Oregon University and World Health Organisation