Methoprene is a compound which mimics the action of an insect growth regulation hormone. It is used as an insecticide because it interferes with the normal maturation process. In a normal life cycle, an insect goes from egg to larva, to pupa, and eventually to adult. Methoprene artificially stunts the insects' development, making it impossible for insects to mature to the adult stages by inhibiting the moulting process this is especially desirable at or before the adult moult, and thus preventing them from reproducing. I hope the insects don't think of the same thing for us...??!
Anyway, for it to be any use, it must be administered at the proper stage of the target pest's life cycle. Methoprene is not toxic to the pupal or adult stages. Treated larvae will pupate but adults do not hatch from the pupal stage. Methoprene is also considered a larvicide since it is effective in controlling the larval stage of insects. Methoprene is used in the production of a number of foods including meat, milk, eggs, mushrooms, peanuts, rice, and cereals. It is also used in aquatic areas to control mosquitoes and several types of ants, flies, lice, moths, beetles, and fleas. It is slow acting and should be viewed as a long-term control measure. It is useful for the control of; Coleoptera, Siphonaptera, Diptera, Formicidae and Homoptera. As it is used in aquatic environments, for mosquito control, it must also be understood that methoprene is highly toxic to aquatic invertebrates and moderately affects fish. There are deformities in frogs and many scientists working on the deformed frog problem believe that the cause is a chemical one. One of the most likely chemical candidates for causing such deformities is methoprene, a synthetic insect growth hormone (IGH) that is sprayed in problem areas in order to control pests such as mosquitoes.
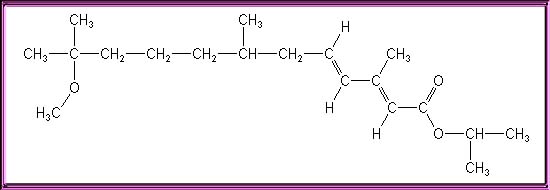
Pictures may vary due to rotation about single bonds
Chemical Formula: C19H34O3
CAS Number: 40596-69-8
Other Names: ispropyl(E,E)-(R,S)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate. Isopropyl (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate; 2,4-Dodecadienoic acid (E,E)-11-methoxy-3,7,11-trimethyl-, 1-methylethyl ester; ZR-515; Altosid SR-10 and CP-10; Apex 5E; Diacon; Dianex; Kabat; Minex; Pharorid; Precor; Altosid CP-10; Altosid SR-10; 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate, (E,E)-; Altosid; 2,4-Dodecadienoic acid, 11-methoxy-3,7,11-trimethyl-, 1-methylethyl ester; Altosid briquets; Dodecadienoic acid, 11-methoxy-3,7,11-trimethyl-, isopropyl ester, (E,E)-; Isopropyl (E,E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate; Manta; Methylethyl (E,E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate;
Trade Names: Altosid , Apex, Diacan, Dianex, Kabat, Minex, Pharorid, Precor, ZR and Pharoid to name a few.
Appearance: Technical methoprene is an amber or pale yellow liquid with a faint fruity odour, esters tend to have a fruity odour.
Physical Properties: Melting point = 164°C; Boiling Point = 135 - 136°C; Molecular Weight: 310.48; Water Solubility: 1.4 mg/L @ 25 C; Solubility in Other Solvents: Miscible in organic solvents;
Toxicology: Acute toxicity: Methoprene is practically nontoxic when ingested or inhaled and slightly toxic by dermal absorption. The oral LD50 for methoprene in rats is greater than 34,600 mg/kg, and in dogs is greater than 5000 mg/kg (remember the bigger the number the less the toxicity). It is slightly toxic by skin exposure, with reported dermal LD50 values of greater than 2000 to 3000 mg/kg in rabbits. Methoprene is not an eye or skin irritant, and it is not a skin sensitiser. The inhalation LC50 for methoprene in rats is greater than 210 mg/L. No overt signs of poisoning have been reported in incidents involving accidental human exposure to methoprene.
Chronic toxicity: No methoprene-related effects were observed in 2-year feeding trials with rats given doses of 250 mg/kg/day, nor in mice given 30 mg/kg/day. Liver changes were observed in mice fed 50 to 250 mg/kg/day of methoprene during an 18-month study. Increased liver weights occurred in rats fed 250 mg/kg/day for 90 days, but not during a 24-month feeding study in which rats were fed 125 mg/kg/day.
Effects on birds: Methoprene is slightly toxic to birds. The reported 5- to 8-day LC50 values for Altosid, a methoprene formulation, are greater than 10,000 ppm in mallard ducks and bobwhite quail, and the acute oral LD50 for Altosid is greater than 4640 ppm in chickens. In mallards an acute oral LD50 of greater than 2000 mg/kg was determined. Non-lethal effects that may affect survival of the birds did appear at acute oral doses of 500 mg/kg. These effects appeared as soon as 2 hours after treatment and persisted for up to 2 days and included slowness, reluctance to move, sitting, withdrawal, and lack of coordination. These effects may decrease bird survival by making them temporarily more susceptible to predation. No effects were observed in the reproduction of bobwhite quail and mallard ducks at 30 ppm constant feeding of Altosid.
Effects on aquatic organisms: Methoprene is slightly to moderately toxic to fish. The reported 96-hour LC50 values for the methoprene formulation Altosid were 4.6 mg/L in bluegill sunfish, 4.4 mg/L in trout, and greater than 100 mg/L in channel catfish and largemouth bass. Methoprene residues may have a slight potential for bio-concentration in bluegill sunfish and crayfish. Methoprene is very highly toxic to some species of freshwater, estuarine, and marine invertebrates, while the acute LC50 values are greater than 100 mg/L in freshwater shrimp, and it is greater than 0.1 mg/L in estuarine mud crabs. Altosid had very little effect, if any, on exposed non-target aquatic organisms including waterfleas, damselflies, snails, tadpoles, and mosquito fish. There are indications that methoprene will affect amphibians, and a lot of research is going on in these areas.
Effects on other organisms: Tests with earthworms showed little if any toxic effects on contact. It is nontoxic to bees.
References: EXTOXNET primary files maintained and archived at Oregon State University, and other sources.